Airway Surface Liquid Volume Regulation
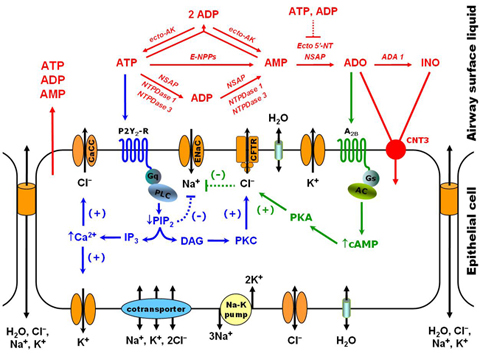 Our research project is aimed at describing how lung epithelial cells regulate mucus hydration. As a protection against lung infection, inhaled pathogens trapped in the airways are removed by mucus transport towards the digestive tract. This physiological process, named mucociliary clearance, depends on proper mucus hydration. The importance of this clearance process is illustrated by the disease Cystic Fibrosis (CF), where absence of a single gene (CFTR) causes mucus dehydration, reduced clearance and persistent lung infection. Lung epithelial cells regulate mucus hydration by controlling ion movement across cell membranes; water flows as a response to changes in the osmotic pressure. We are building a computational model that describes the main events at the cellular level that regulate mucus hydration. The model takes into account the three main ions that contribute to osmotic pressure (Na+, K+, Cl-), ion movement due to electrochemical gradients, and the regulation of ion channels by extracellular signals (ATP and adenosine). Model parameters are fitted to reproduce bioelectric measurements performed in cell cultures of human airway epithelia.
Our research project is aimed at describing how lung epithelial cells regulate mucus hydration. As a protection against lung infection, inhaled pathogens trapped in the airways are removed by mucus transport towards the digestive tract. This physiological process, named mucociliary clearance, depends on proper mucus hydration. The importance of this clearance process is illustrated by the disease Cystic Fibrosis (CF), where absence of a single gene (CFTR) causes mucus dehydration, reduced clearance and persistent lung infection. Lung epithelial cells regulate mucus hydration by controlling ion movement across cell membranes; water flows as a response to changes in the osmotic pressure. We are building a computational model that describes the main events at the cellular level that regulate mucus hydration. The model takes into account the three main ions that contribute to osmotic pressure (Na+, K+, Cl-), ion movement due to electrochemical gradients, and the regulation of ion channels by extracellular signals (ATP and adenosine). Model parameters are fitted to reproduce bioelectric measurements performed in cell cultures of human airway epithelia.
Regulation of Extracellular Nucleotides by Airway Epithelia
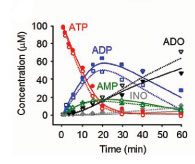
In the airways, adenine nucleotides support a complex signaling network mediating host defenses. Released by the epithelium into the airway surface liquid (ASL), adenine nucleotides are sensed by membrane receptors (P2Y2 and A2b), which trigger a cascade of intracellular signals that regulate ciliary beat frequency, mucus release, mucus hydration, and mucociliary clearance. The importance of this signaling pathway in lung pathophysiology, particularly in cystic fibrosis, has led us to develop a mathematical model of how airway epithelia regulate extracellular nucleotides. The mathematical model describes nucleotide release, metabolism by ecto-enzymes into nucleosides (Adenosine and Inosine), and nucleoside uptake by membrane transporters (CNT3).
Sandefur CI, Boucher RC, Elston TC. Mathematical model reveals role of nucleotide signaling in airway surface liquid homeostasis and its dysregulation in cystic fibrosis. Proc Natl Acad Sci U S A. 2017 Aug 29;114(35):E7272-E7281. doi: 10.1073/pnas.1617383114. Epub 2017 Aug 14.
Garcia GJ, Boucher RC, Elston TC. Biophysical model of ion transport across human respiratory epithelia allows quantification of ion permeabilities. Biophys J.2013 Feb 5; 104(3):716-26.
(Pubmed | Journal)
Garcia GJ, Picher M, Zuo P, Okada SF, Lazarowski ER, Button B, Boucher RC, Elston TC. Computational model for the regulation of extracellular ATP and adenosine in airway epithelia. Subcell Biochem. 2011; 55:51-74.
(Pubmed | Journal)
Zuo, P., Picher, M., Okada, S.F., Lazarowski, E.R., Button, B., Boucher, R.C., and Elston, T.C. (2008). Mathematical model of nucleotide regulation on airway epithelia. Implications for airway homeostasis. J Biol Chem 283, 26805-26819.Zuo P, Picher M, Okada SF, Lazarowski ER, Button B, Boucher RC, Elston TC. Mathematical model of nucleotide regulation on airway epithelia: Implications for airway homeostasis. J Biol Chem. 2008 Sept 26; 283(39):26805-26819
(Pubmed | Journal)